Background: Experience with ceftazidime-avibactam (CAZ-AVI) for bacteremia caused by carbapenem-resistant Enterobacterales (CRE) and Pseudomonas aeruginosa (CRPA) in hematological patients is limited.
Methods: We performed a single-center, retrospective, observational study including patients receiving CAZ-AVI for bacteremia due to CRE or CRPA between 2018 and 2022. The primary outcome was 30-day survival. To identify predictors of survival, multivariable analysis was performed.
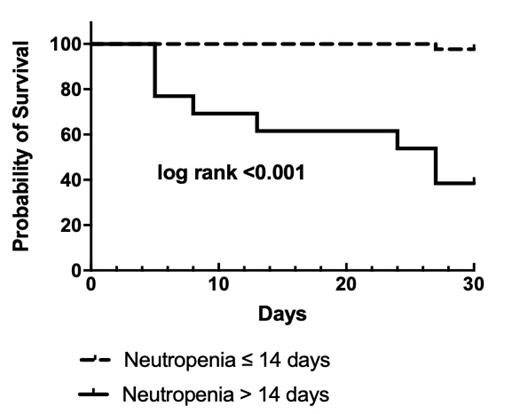
Results: 56 patients were included and 57 (41 CRE and 16 CRPA) strains were isolated. 35 strains produced carbapenemase (25 MBL, 10 serine-beta-lactamase). 48 (85.7%) patients received combination therapy. All patients with MBL-CRE bacteremia (n=24) received combination therapy with aztreonam. The susceptibility rates to CAZ-AVI were only 25.0% (10/40) in CRE and 80.0% (8/10) in CRPA. The 30-day survival rates were 85.0% (34/40) and 81.3% (13/16) in CRE and CRPA group, respectively. In patients with MBL-CRE bacteremia, 30-day survival was high to 91.7% (22/24) due to combination with aztreonam. Ceftazidime did not influence the activity of aztreonam-avibactam against MBL-CRE in-vitro. Multivariable cox analysis revealed neutropenia > 14 days (P=0.002, HR: 0.029, 95%CI: 0.003~0.260) and higher Pitt bacteremia score (P=0.005, HR: 0.482, 95%CI: 0.291~0.798) were risk factors of 30-day survival.
Conclusions: CAZ-AVI is highly effective for bacteremia due to CRPA and serine-beta-lactamase CRE. Avibactam in combination with aztreonam is highly active for bacteremia due to aztreonam-resistant MBL producers.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal